Hello, members of OT.
Out of pure curiosity, I've been trying to find any specific information that might be of help to the question I ask in the title. I know it might seem dumb at first glance, and it may be so, but I can't help but wonder:
Do molecules of a liquid (not a crystal, so any liquid with isotropic properties), e.g. water, form any type of particular temporary patterns?
In the particular case of water, being a compound of polar bonds, I wonder if molecules in the liquid state would favour forming localised crystalline, albeit not lasting, repeated patterns like the ones one sees in crystalline solid water.
Thank you in advance for entertaining my silly questions.
-
2015-08-12, 08:30 PM #1Pit Lord


- Join Date
- Mar 2014
- Posts
- 2,328
Does water, or any other non-crystalline liquid form patterns between its molecules?
-
2015-08-12, 08:33 PM #2
In general, when patterns are formed it's because the liquid is changing states.
It is by caffeine alone I set my mind in motion. It is by the beans of Java that thoughts acquire speed, the hands acquire shakes, the shakes become a warning.
-Kujako-
-
2015-08-12, 08:48 PM #3
http://www.abc.net.au/science/articl...24/3374497.htm
Supercooled water forms "tetrahedron shapes" if kept from nucleation.
-
2015-08-12, 08:57 PM #4Pit Lord


- Join Date
- Mar 2014
- Posts
- 2,328
-
2015-08-12, 09:24 PM #5
Yes, there is short-range ordering of molecules in liquid water, like you suspected. Small hydrogen-bonded assemblies of water molecules keep forming up and then breaking down. One can implicitly see the presence of this ordering in the experimental entropies of vaporization:
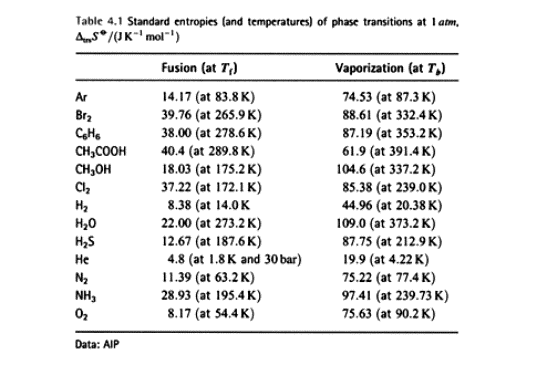
Entropy is, kind of, a measure of "disorder" in the system; entropy of vaporization means the change in system entropy when liquid changes into gas. Upon vaporization the entropy increases since gas is "more disordered than liquid". Trouton's rule says that for "normal" liquids this change should be around +87 J / (K*mol). However, for water it is much higher than that: +109 J / (K*mol).
This can mean either that water vapor has an unusually high disorder (which is not very plausible) or that liquid water has unusually high ordering (= low disorder). The latter can be explained by the association of water molecules due to hydrogen bonding.Last edited by Uzkin; 2015-08-13 at 08:33 AM.

 Recent Blue Posts
Recent Blue Posts
 Recent Forum Posts
Recent Forum Posts
 Season 4... Just old dungeons and new ilvl?
Season 4... Just old dungeons and new ilvl? [WeakAura] Tombstone's Conditions
[WeakAura] Tombstone's Conditions Did Blizzard just hotfix an ilvl requirement onto Awakened LFR?
Did Blizzard just hotfix an ilvl requirement onto Awakened LFR? MMO-Champion
MMO-Champion


 Reply With Quote
Reply With Quote




